Introduction
The President of the Federal Republic of Nigeria signed into law the National Biosafety Management Agency Bill 2015 on the 18th of April 2015, after it was passed by the National Assembly. The Biosafety law is to domesticate the Cartagena Protocol on Biosafety and to provide a Legal and Administrative Framework for the practice and domestication of Modern Biotechnology and ensure the safe use of genetically modified organisms in Nigeria. The law includes the establishment of a National Biosafety Management Agency to manage the Act. It is also set out to provide a regulatory framework to reconcile the respective needs of international trade and environmental protection with respect to a rapidly growing global modern biotechnology industry.
The Agency was established through the National Biosafety Management Agency Act 2015. It is an Agency of the Federal Ministry of Environment. The Agency is charged with the responsibility for providing a regulatory framework, institutional and administrative mechanisms for safety measures in the application of modern biotechnology in Nigeria with the view to preventing any adverse effect on human health, animals, plants, and environment. The establishment of the Agency is a sign of Nigeria commitment to Global Biodiversity Conservation and Biosafety.
The National Biosafety Management Agency is presently made up of six (6) major departments and ten (10) units as illustrated in the organogram below
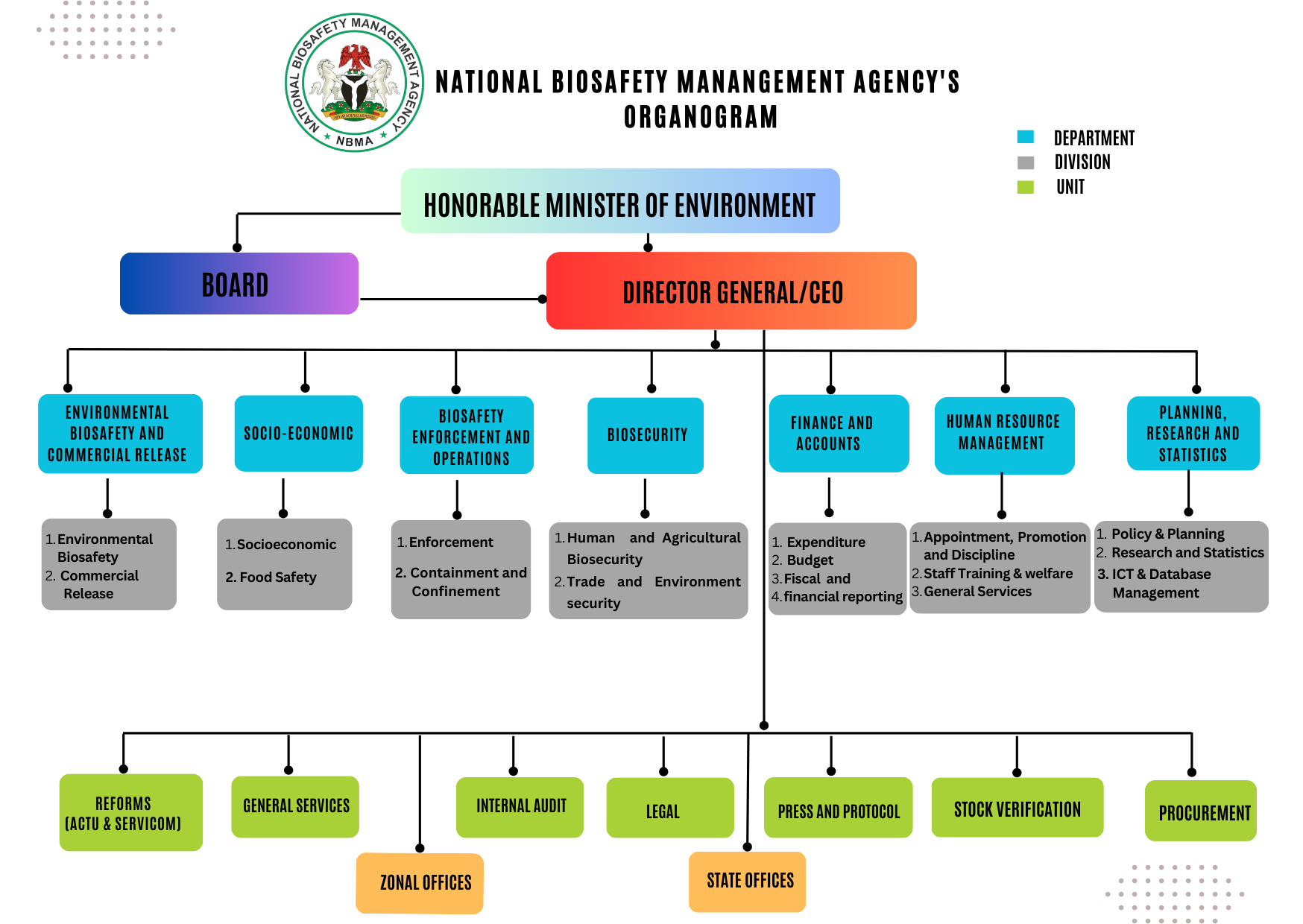
Organogram
The details of the NBMA’s functions specified in part II of the Act are as follow:
- To formulate overall policy guidance on issues of Biosafety in Nigeria.
- Implement the provisions of the Conventions and the Protocols on matters relating to genetically Modified Organisms.
- Render reports to the Secretariat of the Convention on the implementation of the Convention and Protocol on a matter relating to the use of Genetically Modified Organisms.
- Develop measures and requirements and criteria for Biosafety risk assessment peer review and decision making.
- Develop measures and requirements for Biosafety risk assessment.
- Develop risk management plan and strategy for protecting human health, biological diversity, and the environment from potential risks associated with genetically modified organisms.
- Accept and verify applications in respect of genetically modified organisms and keep records of all approvals and unapproved applications as contained in Part VIII. Subsection 24 (1).
- Take samples and carry out laboratory analysis of crops, products or materials for purposes of determining if they contain genetically modified organisms and ensure compliance with this Act.
- Carry out actions necessary to ensure compliance with the legal obligations set out in the Act, including, but not limited to, the inspection of facilities, conduct research activities with genetically modified organisms covered by the Act, the collection and analysis of samples of materials covered by the Act, the monitoring of human health and the environment to determine the effects of genetically modified organisms regulated by the Act.
- Liaise with the secretariat of the convention and the Biosafety clearinghouse with respect to the administrative functions required under the Protocol.
- Carry out and maintain inventory of laboratories with physical and human capacities to conducts research in modern biotechnology.
- Monitor the activities of institutional committees and Biosafety officers.
- Build, equip, and maintain offices and premises for the performance of its action under the Act.
- Pay remuneration, allowances, expenses, and any other benefit to members of the Board and employees of the Agency or any other persons, in accordance with the National Salaries, Income and Wages Commission.
- Carry out capacity-building activities.
- Perform other duties as may be necessary for the full discharge of its functions under this Act, and
- Partner with other relevant local and international agencies for the speedy realization of the Agency’s mandate.
The Act has opened new ground for diversification of the Nigerian Economy in line with the Change Agenda of the present Administration which is aimed at enhancing the socio-economic development of the country. Some of the benefits to be derived by Nigeria under the enabling biosafety law include safety in the practice of modern biotechnology for:
- Increased productivity and improvement in the agricultural sector leading to accruing more income to farmers and enhanced national economic prosperity; creation of jobs and wealth;
- Availability of raw materials for industrial development, especially in the Nigerian textile sector.
- Freedom to innovate safely in the modern biotechnology sector.
- Production of plants or organisms that can reduce the impact of climate change and assist pollution remediation.
- Improvement in medical sector.
- Increased foreign investment earnings from safe modern biotechnology sector.
- Environmental safety and sustainability.
JUSTIFICATIONS FOR THE CREATION OF THE DEPARTMENTS
The National Biosafety Management Agency (NBMA) Act 2015 empowers the NBMA to formulate overall policy guidance on issues concerning Biosafety in Nigeria and to implement the National Biosafety Management Agency Act. The Agency reconciles the need for the safety of Genetically Modified Organisms (GMOs) in international and national trade as well as biodiversity conservation in order to enhance a rapidly growing modern biotechnology industry in Nigeria for the enhancement of the Nigerian Economy. Essentially, the Agency is charged with responsibility for providing a regulatory framework, institutional and administrative mechanisms for safety measures in the application of modern biotechnology in Nigeria, with the view to preventing any adverse effect on human health, animals, plants, and environment.
The Agency in addition to its functions listed in part II of the National Biosafety Management Agency (NBMA) Act 2015, regulates activities of Agencies/Institutes like the National Biotechnology Development Agency, National Cereal Research Institute, Badeggi, Agricultural Research Institute, Zaria, National Root Crops Research Institute, Umudike, Nigeria Institute for Oil Palm Research, Veterinary Research Institute, Jos, Universities, Local and International Companies and organizations that deal on Genetically Modified Organisms (GMOs) etc. The Agency approves and monitors the release of GMOs and ensures safety to the environment and human health.
The scope of the NBMA Act was enlarged through the amendment of the NBMA Act in 2019 to regulate the emerging aspects of modern biotechnology and biosecurity in Nigeria.
As a regulatory Agency that scrutinizes the activities of these various bodies listed above, it is therefore imperative for the Agency to have a good number of Departments and Units to enable it adequately monitor and guide its activities, with a view to ensuring the safety of human health, animals, plants and environment in Nigeria.
DEPARTMENTS
Part III (4) of the Act empowered the NBMA to have such departments as it may deem appropriate (see a copy of the Act and a gazette).
The Departments of the Agency as reflected in the Organogram and their functions are as follows:
- Department of Environmental Biosafety and General Release
- Department of Biosafety Enforcement and Operations
- Department of Socio-Economic and Food Safety
- Department of Planning, Research and Statistics
- Department of Administration and Finance
- Department of Biosecurity

Environmental Biosafety and General Release
The Functions of this Department include:
- Commercial/General release
- Biosafety Risk Assessment and Management of Commercial/General release
- Receipt and processing of the application of Genetically Modified Organisms for Commercial and Open release
- Records of all approvals and unapproved applications for commercial and open releases
- General Environmental Safety
- Develop measures, requirements and criteria for risk evaluation, peer review and decision-making
- Develop measures and requirements for risk assessment and environmental impact assessment
- Develop risk management plan and strategy for protecting biological diversity and the environment from potential risks associated with genetically modified organisms
- Monitoring of the environment to determine the effects of genetically modified organisms regulated by the Biosafety Act
- Liability and Redress
- Carry out such other duties as may be necessary for the full discharge of the functions of the National Biosafety Management Agency under the Act

Biosafety Enforcement and Operations
The Functions of this Department include:
- Unintentional Release of tracking of GMOs
- Emergency issues
- General Enforcement;
- Carry out actions necessary to ensure compliance with the legal obligations set forth in the Biosafety Act, including but not limited to the inspection of facilities, conducting activities with genetically modified organisms covered by the Biosafety Act, the collection and analysis of samples of materials covered by the Act
- Carry out such other duties as may be necessary for the full discharge of the functions of the National Biosafety Management Agency under the Act
- Inspections

Socio-Economic and Food Safety
The Functions of this Department include:
- Socio-Economic, ethical Considerations
- Assessment of socio-economic impacts
- Civil matters
- Develop risk management plan and strategy for protecting human health from potential risks associated with genetically modified organisms
- Monitoring of human health to determine the effects of genetically modified organisms regulated by the Biosafety Act
- Relates with NAFDAC
- Allergenicity, Toxicity
- GM food safety
- Establish safety guidelines for genetically modified foods, feeds and for processing

Planning Research and Statistics
The Functions of this Department include:
- Propose for approval the overall policy guidance on issues of biosafety in Nigeria
- Board matters
- Research and Planning
- Public awareness and enlightenment programs on Biosafety
- Serves as the Secretariat of the National Biosafety Committee
- Maintains a database on releases of GMOs in the country
- Carry out capacity building activities
- Biosafety Treaties, Bi-lateral/Multilateral land Conventions: (Matters on the Convention on Biological Diversity Cartagena Protocol on Biosafety, Supplementary Protocol on Biosafety, the biosafety clearing house, African Union on Biosafety and ECOWAS Biosafety)
- Liaise with relevant stakeholders i.e. civil societies on Biosafety matters
- Serves as the Secretariat of the National Biosafety Committee Liaise with the Secretariat of the Convention and the biosafety clearing house with respect to the administrative functions required under the Protocol
- Maintains a database on releases of GMOs in the country
- Maintain the BCH website and Biosafety DataBase
- Carry out such other duties as may be necessary for the full discharge of the functions of the National Biosafety Management Agency under the Act

Administration and Finance
The Functions of this Department include:
- All functions of the Human Resource Department plus:
- Advising and guiding the management of the Agency on financial matters
- Maintaining the Agency’s Accounts with the Central Bank of Nigeria and Commercial Banks
- Collection of revenues on behalf of the Federal Government and ensuring same is remitted to the appropriate authorities (i.e the sub treasurer of the Federation and the Federal Inland Revenue Service)
- Rendering timely statement of transcripts, Bank Reconciliation and other financial statements to the office of the Accountant-General of the Federation
- Preparation, implementation and monitoring of Budget
- Liaising with the Federal Ministry of Finance and Office of the Accountant-General of the Federation and the National Assembly on matters relating to the Agency’s budget and finance

Biosecurity
The Functions of this Department include:
- Coordinate activities of all relevant MDAs for affective and prompt responses to biosecurity threats including precautionary and regulatory measures in line with the best international practices
- Ensure the control and management of pests, diseases and weeds affecting agriculture and the environment
- Participate in the management of all established Biobanks by performing an audit of institutions and locations with dangerous pathogens and toxin and developing consolidation plan and strategies
- Coordinate biosecurity emergencies by leading the development of multisectoral Biosafety and Biosecurity, incidenst and emergency response strategies and bioterrorism response plans critical for addressing biosecurity events
- Prepare and develop Biosecurity regulatory instruments

Units
Units in the Agency include:
- Legal Unit
- Press and Protocol Unit
- Procurement Unit
- Reform/SERVICOM, Anti-Corruption and Stock Verification Units
- Audit Unit
- Biosafety Intelligence Unit
- Information, Technology & Communication (ICT) Unit
